LPS: A Hidden Variable with Major Consequences in Murine Infection Models
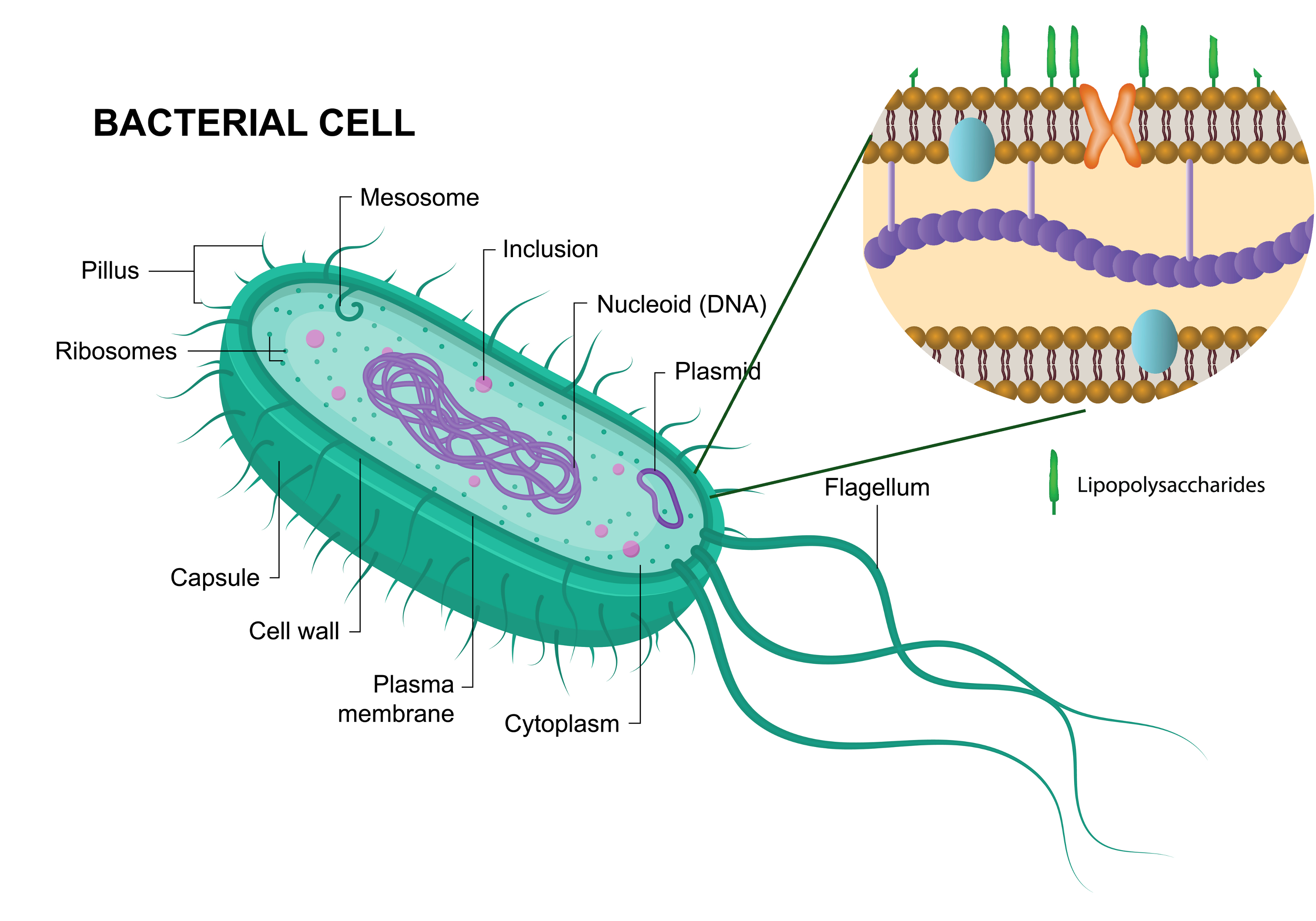
In the realm of animal models used for studying infectious diseases, the mouse has established itself as a methodological standard. Its ease of breeding, manipulable genome, and the wealth of available data make it a valuable sentinel in preclinical research. Yet, at the heart of these studies, a discreet but powerful molecule can undermine results: lipopolysaccharide, or LPS.
LPS: A Key Immune Mediator… and Source of Ambiguity
LPS is a major component of the outer membrane of Gram-negative bacteria. Recognized as a pathogen-associated molecular pattern (PAMP) by Toll-like receptors (TLR4), it triggers a rapid and intense innate immune response. In mice, LPS injection is a classical tool for inducing endotoxemia, mimicking early phases of bacterial sepsis.
What Is Endotoxemia?
Endotoxemia refers to the presence of lipopolysaccharides (LPS)—a major component of the outer membrane of Gram-negative bacteria—in the bloodstream. This condition can occur during systemic bacterial infections, or experimentally through LPS injection in animal models. In mice, endotoxemia typically triggers a rapid and intense systemic inflammatory response, mediated by activation of Toll-like receptor 4 (TLR4) on innate immune cells such as macrophages and dendritic cells.
The result is a cytokine storm involving TNF-α, IL-1β, IL-6, and other mediators that lead to fever, hypothermia, vasodilation, hypotension, and in severe cases, disseminated intravascular coagulation (DIC) and multi-organ failure. This makes LPS both a powerful experimental tool and a source of experimental bias, especially in infection models where LPS release is not controlled and can confound interpretation of treatment efficacy or toxicity.
In preclinical studies, the challenge is to distinguish between the pathological effects of the infection itself and those triggered purely by LPS-mediated immune activation, particularly when assessing novel antimicrobial therapies that cause bacterial lysis.
Underestimated Experimental Interference
In therapeutic evaluation, LPS can act as a major confounding variable. It interferes with:
Survival parameters, by causing deaths unrelated to pathogen activity but due to excessive host-response;
Cytokine profiles, complicating the assessment of anti-inflammatory or pro-resolving drug-effects;
Behavioral phenotypes, where LPS-induced neuroinflammation causes hypoactivity, anorexia, or social withdrawal, often misinterpreted as drug side effects.
Moreover, LPS disrupts the intestinal barrier, alters the microbiota, and may facilitate colonization by opportunistic pathogens, as shown in recent studies on E. coli and Klebsiella. link
The Mouse: A Hypersensitive Model
While the mouse is one of the most commonly used models in preclinical infectious disease research, it shows well-documented hypersensitivity to LPS compared to humans. A dose of 0.5 to 1 mg/kg LPS via intraperitoneal injection can trigger hypothermia, lethargy, significant weight loss, and, at higher doses (≥5 mg/kg), endotoxin shock with rapid death.
This heightened sensitivity stems partly from differences in Toll-like receptor expression (TLR4/CD14/MD-2), strong macrophage reactivity, and rapid cytokine release kinetics (TNF-α, IL-1β, IL-6). This profile makes mice particularly vulnerable to unintended or treatment-induced LPS release.
Factors Influencing Murine Response to LPS:
Genetic background:
C57BL/6: strong pro-inflammatory response;
BALB/c: milder reaction;
C3H/HeJ: TLR4 mutants, LPS-resistant.
Sex and age:
Young females typically show less intense responses than adult males;
Aging amplifies the inflammatory response.
Diet:
High saturated fat intake increases LPS sensitivity (via metabolic endotoxemia).
Gut microbiota:
Dysbiosis promotes endogenous LPS translocation.
Housing conditions:
Stress, subclinical infections, and circadian rhythm impact response.
These factors make mice a biologically sensitive yet unstable system, in which LPS acts as a catalyst for confounding effects.
Recommendations for model interpretation
Given the biases that LPS can introduce, a rigorous strategy is needed to ensure result reliability:
Include LPS control groups
Add a “LPS-only” arm (at comparable doses to those expected from bacterial lysis) to isolate treatment effects from endotoxin response.
Quantify key cytokines
Measure TNF-α, IL-6, IL-1β, CXCL1, etc., in plasma and target tissues.
Standardize animal parameters
Unify strain, sex, age, weight, injection timing, and housing conditions.
Use neutralizing agents or encapsulation strategies
Polymyxin B, LBP, or nanoparticles to limit LPS release.
Apply multiparametric behavioral analysis
Go beyond survival: include clinical scoring, locomotor activity, food intake, thermoregulation, and cognitive tests.
Cross-validate with ex vivo or multi-species models
Organoids, explants, and human in vitro models to confirm observations.
LPS is at once a danger signal, a modeling tool, and a potential experimental artifact. Managing it effectively in murine infection models is key to avoiding false positives and negatives. Recognizing its role is essential to ensure the predictive value of preclinical research.
Vibiosphen’s Expertise in Preclinical Models
At Vibiosphen, we specialize in advanced preclinical models for infectious diseases, including complex in vivo murine systems. Our scientific team is highly trained in managing the intricacies of endotoxemia and LPS-related responses. We implement rigorous controls, dosing strategies, and immune monitoring to distinguish true therapeutic effects from LPS-driven artifacts.
From phage therapy to endolysin development and antibiotic efficacy studies, we tailor our models to ensure high translational relevance and immunological fidelity, always accounting for the powerful role of LPS in infection dynamics.
With Vibiosphen, your preclinical studies benefit from scientific precision and biological insight—ensuring that every variable, even the invisible ones, is under control.
📚 Key References:
Cieślik M. et al. Animal Models of Phage Therapy, Microorganisms (2021)
Langdon A. et al. Sublethal LPS enables gut-luminal pathogen expansion, Cell Host Microbe (2025)
Garre M. et al. Behavioral effects of systemic LPS in mice, Int Immunopharmacol (2024)
Briers Y. et al. Engineered Endolysins and LPS toxicity, mBio (2014)
Catégories
Pagination
- Page précédente
- Page 2
Archives
- juin 2025 (3)
- mai 2025 (1)
- mars 2025 (1)
- mai 2024 (1)
- avril 2024 (2)
- septembre 2023 (1)
- août 2023 (1)
- mai 2023 (1)
- avril 2023 (2)
- février 2023 (1)
- décembre 2022 (1)
- octobre 2022 (1)
- juin 2022 (1)
- mai 2022 (3)
- avril 2022 (1)
- février 2022 (2)
- janvier 2022 (3)
- décembre 2021 (2)
- novembre 2021 (1)


