Interaction compound-gut Microbiota
An oral treatment can potentially interact with the gut microbiota. It is therefore imperative to add a validation level for drug candidates before the clinical phases, to ensure a limited drug / microbiota interaction.
Gut microbiota and drugs interaction
The human gut microbiota is now associated with drug responses and efficacy, while the chemical compounds included in these drugs can also have a impact on intestinal bacteria.
80% of R&D budgets are dedicated to clinical trials (Association for the British Pharmaceutical Industry, Lopez JC (2020)). The average time to the end of clinical Phase II is 5-7 years (LEEM 2020)
The in vitro platform dedicated to the pharmaceutical industry allows in the preclinical phase, in order to eliminate candidate drugs with deleterious effects. This in vitro platform is coupled with an in vivo platform of animal experiments, with gnotobiotic models synchronized with in vitro.

Multiple endpoints analysis
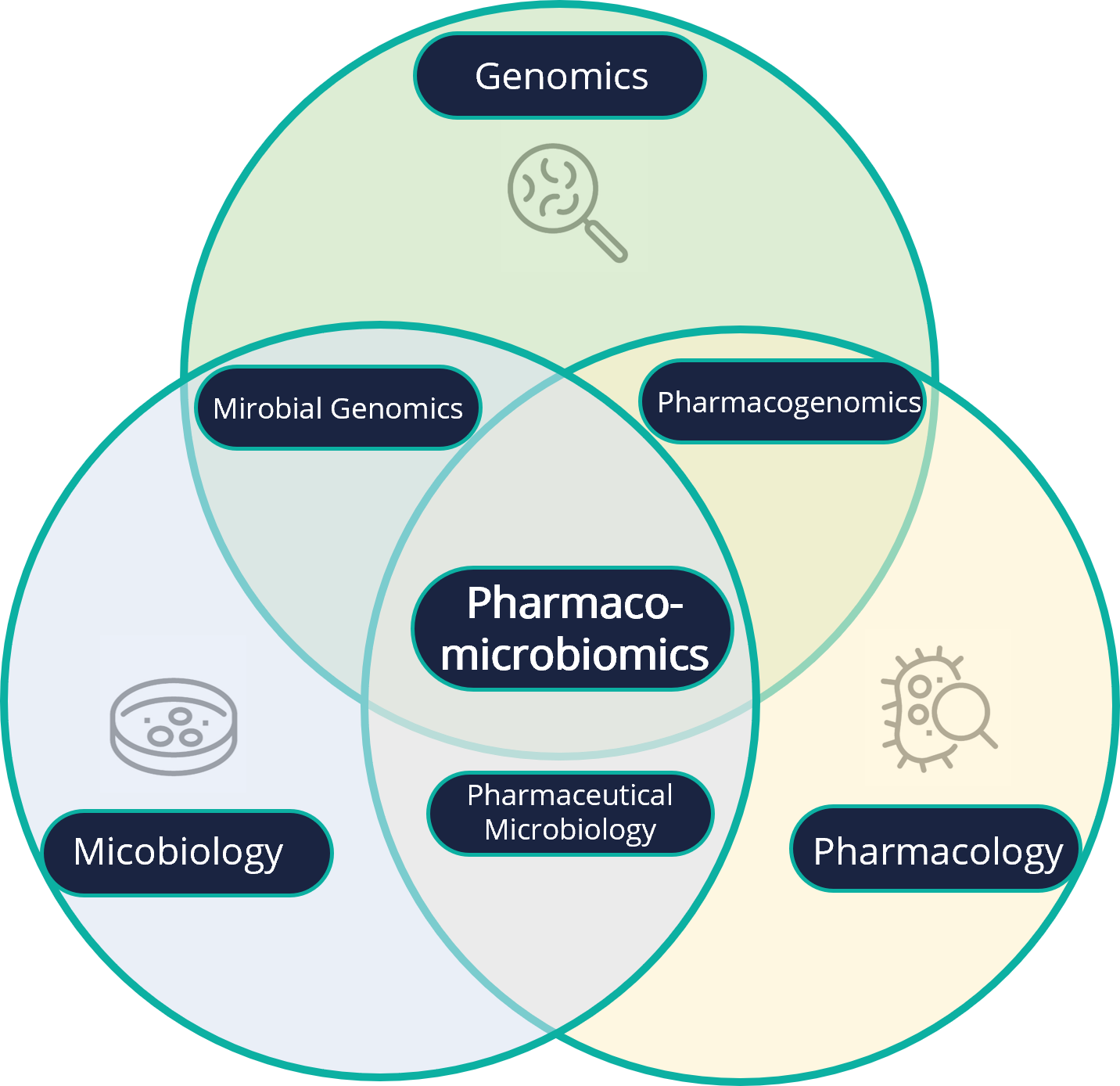
Pharmacomicrobiomics is defined as the study of interactions between a microbiota and a pharmaceutical agent.
Pharmacomicrobiomics is in fact at the intersection of microbiology, pharmacology and genomics
This assay can be implemented during all phases of preclinical research. The purpose of the module is to anticipate the interactions between a drug and a microbiota.
